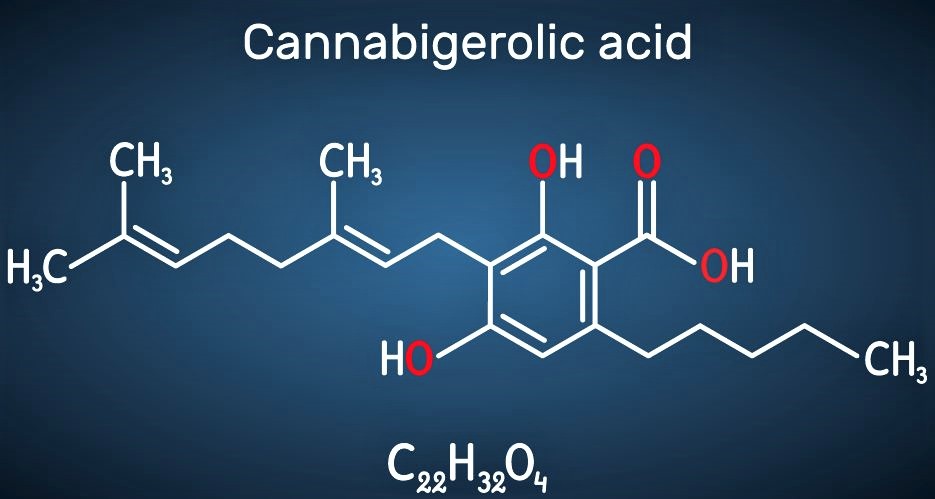
Are Cannabinoids Legal?

History of Cannabinoids in the U.S.
The regulatory environment surrounding cannabis, hemp, and cannabinoids is complicated, to say the least. And while we’ve spent some time defining cannabinoids and diving into their potential benefits with the endocannabinoid system, to really understand these compounds, it’s important to understand the past, present, and future of their regulation.
Cannabis was first made illegal in the United States as part of the Marijuana Tax Act of 1937. The law, which was based on claims that its use led to violent behavior, was later deemed unconstitutional and repealed. But the damage to cannabis’ reputation was done.
In 1961 the United Nations Commission on Narcotic Drugs (CND) classified the cannabis flower and resin on its list of controlled substances. This was followed in the US by the Controlled Substances Act in 1970, which classified cannabis as a Schedule 1 substance. This is the most restrictive category and made it illegal to grow, possess, or use cannabis.
In 1996, California was the first state to allow for the medicinal use of cannabis for people with conditions including Cancer, AIDS, and Glaucoma. Today, the medical use of cannabis is allowed in 33 states, and an additional seven states, including Washington, California, and Colorado have passed laws allowing for the recreational use of cannabis. However, legal use under a state law is not necessarily compliant with U.S. federal law, which adds to the complexity and confusion.
Restrictive laws and unclear regulation have made it difficult for scientists to legally access cannabinoids to study their impact on the human body. And while there’s a lot of debate at the state level and federal level about how to regulate cannabis, one of the most significant pieces of legislation to recently emerge was focused on hemp, a variation of cannabis that does not contain delta-9 tetrahydrocannabinol (delta-9 THC) which is known for its intoxicating effect.
The 2018 US Farm Bill
The US Farm Bill regulates agriculture and food policy for the country and is updated every five years. The 2018 bill included the Hemp Act, which made the commercial production of hemp legal. Hemp by definition cannot contain more than 0.3% delta-9 THC.
The Hemp Act de-scheduled the 100-plus cannabinoids found in hemp (expect for delta-9 THC), meaning they were no longer subject to the Controlled Substances Act. This provided a new, and potentially valuable, crop for U.S. farmers with uses of hemp ranging from paper and clothing to foods and ingredients.
But like many things with the regulation of cannabis, it’s not that simple. Cannabidiol, or CBD, is one of the 100-plus cannabinoids that was descheduled by the 2018 Farm Bill. It is abundant in hemp and is being used in a variety of consumer products. CBD has been shown to have a therapeutic effects for humans, and while it’s not subject to the Controlled Substance Act, it faces its own regulatory challenges within the Food and Drug Administration.
CBD and the FDA
The FDA is the government agency responsible for ensuring the ingredients and products that are used for cosmetics, supplements and food are safe. The agency also approves pharmaceutical products to ensure they are safe and efficacious for the diseases they are designed to treat.
The FDA regulates food, drugs and cosmetics per a set of regulations in the Food Drug and Cosmetics Act (FDCA) that was originally passed in 1938. In the FDCA, there is a specific provision which sits at the center of the issues surrounding CBD as an ingredient in food and supplements.
This provision states that an ingredient cannot become a dietary or food ingredient if it has not legally been in the food supply before being approved as a pharmaceutical or investigational new drug by the FDA.
This is problematic for CBD because in June 2018 the FDA approved the use of CBD in a drug called Epidiolex. The drug is used to treat seizures associated with two rare and severe forms of epilepsy.
Epidiolex was approved six months before the 2018 Farm Bill passed when CBD was still considered a Schedule 1 substance. This means that while CBD is no longer subject to the Controlled Substance Act, it also cannot be used in food and supplement products due to the provision in the FDCA.
The result, like much regulation around cannabinoids is a bit confusing. While CBD is a safe, legal additive in products like skin cream, it is not allowed in products like multivitamins, or chocolate bars.
If you’re confused, you’re not alone. A quick internet search will turn up dozens of food and supplement products featuring CBD, but these products are not compliant with the FDA, and may not comply with regulations that focus on their purity or safety. Buyer beware.
The path forward
The regulatory landscape for cannabis has come a long way since the Marijuana Tax Act of 1937, and it still has a way to go. Demetrix is closely working with federal and state regulators to help drive forward meaningful change to cannabis regulation, while delivering safe, legal, and efficacious products using cannabinoids. By bringing scientific rigor to the study of cannabinoids, we’re helping unlock new health and wellness benefits for millions of people and animals around the world.